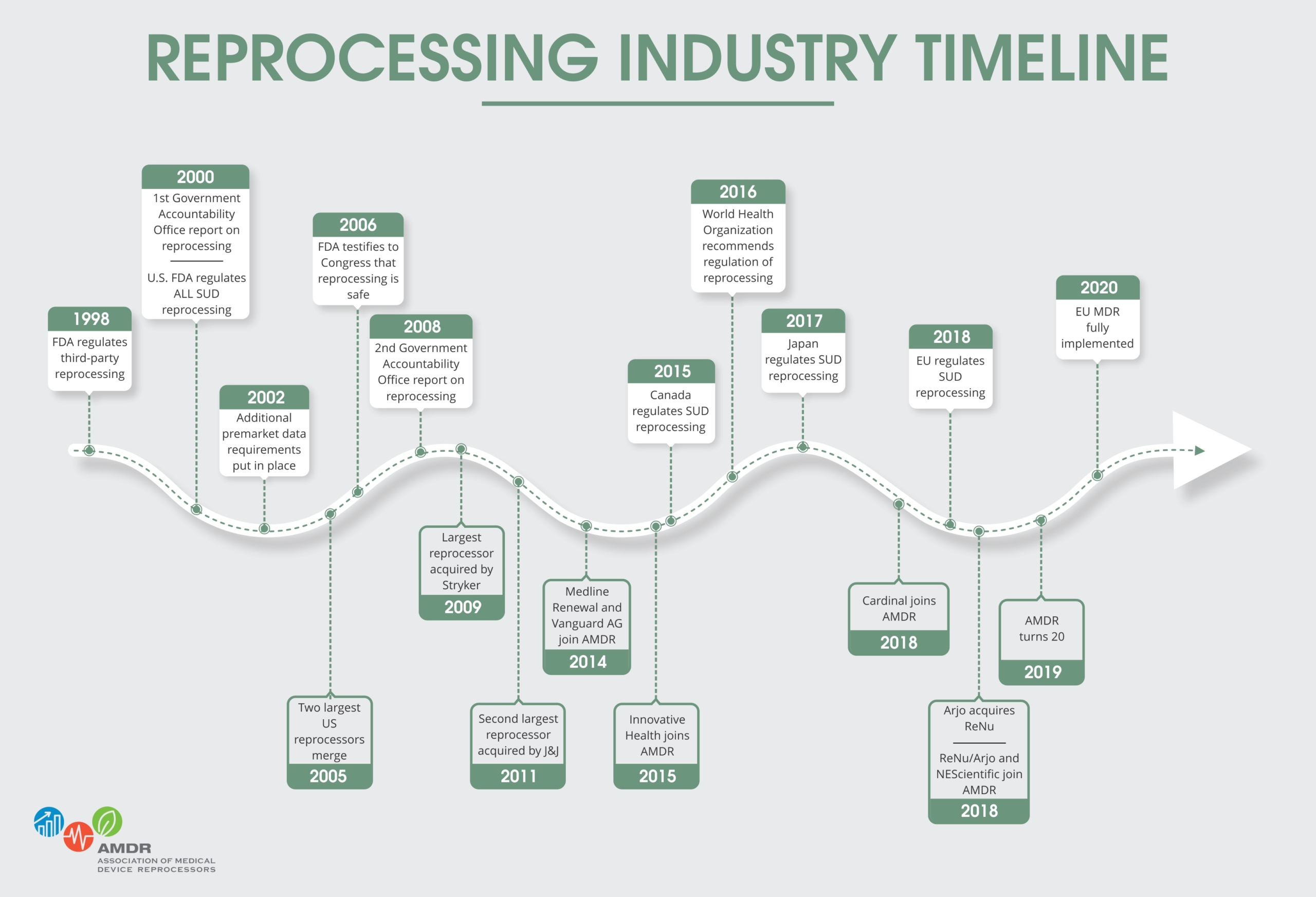
History
The reprocessing of medical devices labeled for “single patient use” has been standard practice in United States hospitals for years. Medical device reprocessing emerged over two decades ago, when original equipment manufacturers (OEMs) began to change the labels on certain devices from “reusable” to “single patient use,” without making significant structural changes in the devices. Hospitals recognized that the “single patient use” label was often motivated by economic objectives, rather than patient safety concerns. Thus, rather than needlessly discarding such devices after one use, hospitals began to reprocess them.
Historically, the majority of reprocessing had been conducted at in-hospital reprocessing centers. In the last decade, however, the commercial reprocessing industry emerged at the request of hospitals that were seeking a way to outsource their reprocessing needs.
Today, commercial reprocessing is a small but expanding industry. A majority of the commercial reprocessing done in the United States today is performed by members of the Association of Medical Device Reprocessors (AMDR).